Moderna Booster Dose
Moderna urges FDA to authorize a half-size booster dose of its Covid-19. At this time the Pfizer-BioNTech booster authorization only applies to people whose primary series was Pfizer-BioNTech vaccine.

Fda Panel Endorses Moderna Booster Shots For Seniors High Risk Groups Komo
Cutting the Moderna doses in half could help reduce the risks of side effects from the booster.

Moderna booster dose. Learn more about the facts behind the COVID-19 Vaccine. A recent study published on the preprint server medRxiv presents evidence that a booster dose of the Moderna vaccine can induce a robust memory immune response against coronavirus disease 2019. 1 day agoThe advisory panel although approving of the Moderna booster shot pointed out the fact there was little data supporting the third dose.
Learn about the vaccine types. But they also pointed out that the. People in the recommended groups who got the Moderna or JJJanssen vaccine may need a booster shot.
Why would the Moderna booster shot be a half dose. Food and Drug Administration to allow the use of a third booster dose of its Covid-19 vaccine. Moderna submitted data to the FDA seeking evaluation for its booster shot on Sept.
Moderna said it chose the lower-dose booster because it triggered fewer uncomfortable shot reactions such as fever and achiness but also leaves more vaccine available for the global supply. So the moderna booerst is going to be a half dose compared to the. Ad Delta variant is the most contagious one weve seen yet.
A vaccination advisory panel for the Food and Drug Administration unanimously endorsed the authorization of emergency approval for the Moderna half-dose booster shot for at-risk Americans. Ad FDA has authorized the Moderna COVID-19 Vaccine under an Emergency Use Authorization. Moderna had pushed for the half-dose shot in its booster application submitted to federal regulators on Sept.
1 day agoLast modified on Fri 15 Oct 2021 0517 EDT US health advisers said on Thursday that some Americans who received Modernas Covid vaccine should. Modernas current vaccine shot is a 100-microgram dose compared with Pfizers 30-microgram dose. Cutting the Moderna doses in half could help.
CDC recommends full vaccination. Modernas current vaccine shot is a 100-microgram dose compared with Pfizers 30-microgram dose. Bloomberg citing people familiar with the matter reported last week that the US.
The FDA is considering booster shots of the Pfizer and BioNTech. The drugmakers original vaccine contains 100 micrograms of mRNA in each shot. Is the moderna booster the same dose as the first two shots.
Moderna argues FDA should authorize half-dose of vaccine as booster Advertising On Thursday advisers are expected to consider data supporting a. Stay safe get all recommended doses for your vaccine type. Moderna on Wednesday asked the US.
FDA reviewers noted that a half-dose booster shot of the Moderna vaccine topped off the level of antibodies the most easily measurable barometer of immunity. So far Americans who received the Moderna COVID-19 vaccine cant get booster shots but could that soon be changing. 1 day agoModerna said it chose the lower-dose booster because it triggered fewer uncomfortable shot reactions such as fever and muscle aches but also leaves more vaccine available for the global supply.
Pfizer for comparison has two 30-microgram doses to start vaccination. More data on the effectiveness and safety of Moderna and JJJanssen booster shots are expected soon. Food and Drug Administration was leaning toward authorizing half-dose booster shots for those who received Modernas two-shot mRNA vaccine.
1 day agoOthers questioned whether half a dose was the right amount and whether a Moderna booster would work better if it was given at least eight. So the booster shot would be a 50-microgram dose. Modernas initial two-dose regime includes two 100-microgram doses.
Pfizers dose on the other hand is the same. Thats a really good question because it is different. We are pleased to initiate the submission process for our booster candidate at the 50 µg dose with the FDA.
Modernas authorization request includes results from a study of 171 people who received the authorized 100-microgram doses of Modernas vaccine and a 50-microgram booster dose at least six months. Moderna had asked for emergency use authorization for a half dose of its vaccine to be used as a booster for certain people. Though the recommendation aligns with the previous one for Pfizer recipients Modernas booster dose is only half the dosage of its first two shots.

Covid Booster Shot Moderna Says Vaccine Generates Promising Immune Response Against Variants
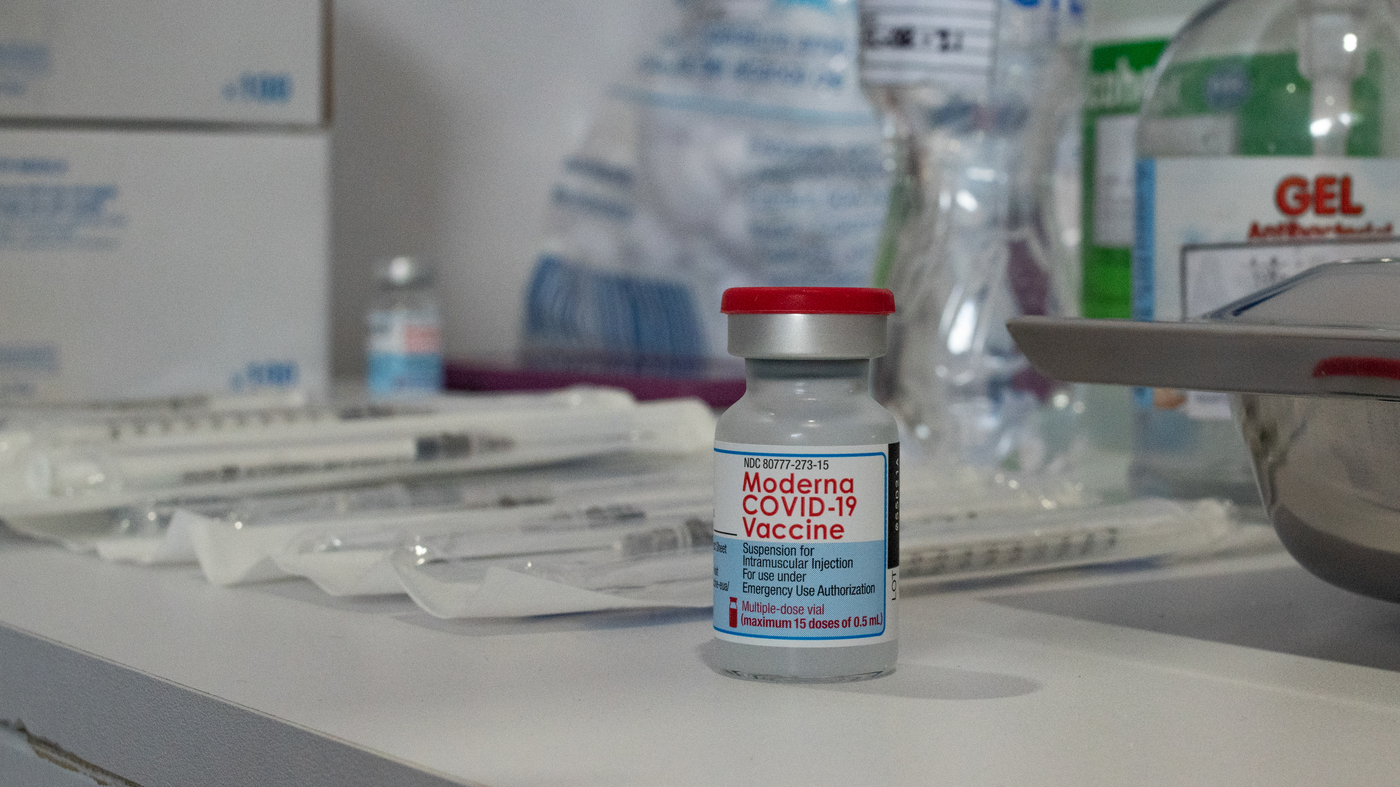
Moderna Asks Fda To Authorize A Booster Shot Of Its Covid 19 Vaccine Coronavirus Updates Npr
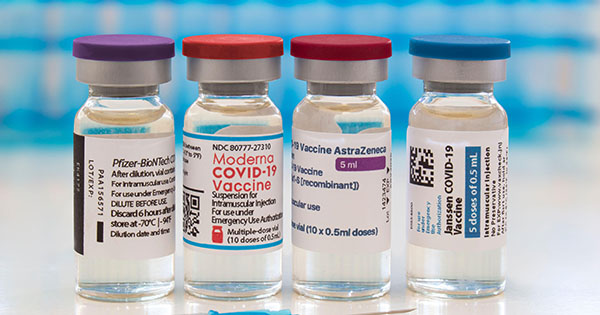
Covid Booster Shots Everything You Need To Know The Brink Boston University

J J Covid Shot Performs Best Paired With Booster Dose From Moderna Or Pfizer Study Suggests
/cloudfront-us-east-1.images.arcpublishing.com/gray/SKUFHPVFMNCM5OJ2V4G4FZKD3U.jpg)
Fda Panel Endorses Lower Dose Moderna Covid Shot For Booster

Fda Panel Endorses Lower Dose Moderna Covid Shot For Booster

Moderna Plans To Have Covid Vaccine Booster Shot Ready By Fall Cbs News
Covid Vaccine Ema Backs Pfizer Or Moderna Booster Shots For People With Weak Immunity Euronews

Fda Panel Endorses Lower Dose Moderna Covid Shot For Booster

Moderna Or Pfizer Booster Works Better For People Vaccinated With J J Study France 24